React
Respond
Reconnect
About Us
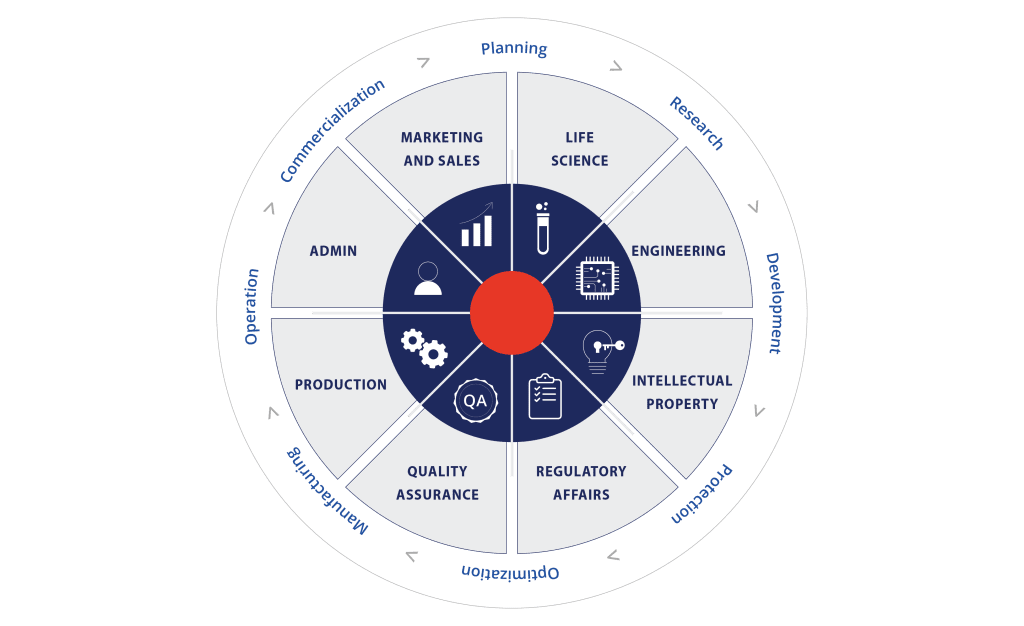
ALiA BioTech is a group of companies that deliver accurate and actionable diagnostic information through our Point-Of-Care Testing (POCT) platform and BioChip, for faster clinical decisions and better economic outcomes for professional and front-line workers.
ALiA BioTech group is formed with our sole purpose of streamlining the way we response to diseases and driving innovations to shape the future of disease diagnostics. ALiA BioTech group has formed a diverse team in Hong Kong, China, France & USA, which is originated from a strong vision for transforming the diagnostic landscape with faster response to disease management. [Sanwa BioTech is a member company of ALiA BioTech group based in Hong Kong. Contact us for collaboration.]